Information for Manufacturers(MED)
The following are the information for manufacturers about the procedures for inspections, auditing and certification for products under the Council Directive 2014/90/EU on marine equipment.
General
At the request of manufacturers, the Piraeus Office of ClassNK (registered in The Hellenic Republic as Nippon Kaiji Kyokai (Greece) S. A., one of the notified bodies recognized by the Government of the Hellenic Republic) (hereinafter referred to as the “Notified Body”), may conduct the conformity assessments in accordance with the requirements of the Council Directive 2014/90/EU on marine equipment for materials and equipment (hereinafter referred to as the “products”) in cooperation with Material and Equipment Department and Certification Department of the Society.
Upon satisfactory completion of these assessments, the manufacturers should affix the Wheel Mark to their products, issue an accompanying EC Declaration of Conformity and place their products on the market.
|
Conformity Assessment Procedures
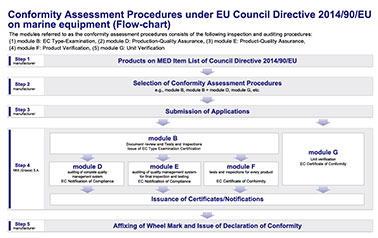
The conformity assessment procedures for products under the Council Directive 2014/90/EU on marine equipment are briefly explained in reference to the flow chart.
Step 1. Products on MED Item List of Council Directive 2014/90/EU
A manufacturer is to ascertain that its products in questions are in the MED Item List “Equipment for which detailed testing standards already exist in International Instruments” in Commission Implementing Regulation of the Council Directive 2014/90/EU on marine equipment
Step 2. Selection of Conformity Assessment Procedures
MED Item List specifies the conformity assessment procedures applicable to individual products.
The manufacturer is to choose the appropriate procedures for conformity assessment among those specified in MED Item List, such as the assessment by means of inspections or auditing, or of the combination of inspections and auditing, bearing in mind the frequency of delivery / quantity of the products manufactured, and other factors.
One example is the combination of inspections and auditing as the conformity assessment procedures for lifeboats as accepted in MED Item List as follows:
- EC Type-Examination (module B) + Production-Quality Assurance (module D)
- EC Type-Examination (module B) + Product Verification (module F)
- Unit Verification (module G)
Step 3. Submission of Applications
The manufacturer is to submit an application for conformity assessment for its products to the Notified Body.
Step 4. Inspections, Auditing and Issuance of certificates/notifications
(1) Initial Inspections and Auditing
It is to be noted that among the conformity assessment procedures is the EC Type-Examination (module B) which is always required to be chosen except where the Unit Verification (module G) is chosen.
The manufacturer who has decided to choose the EC Type-Examination (module B) is to submit an application for the EC Type-Examination (module B) to the Notified Body.
Once the EC Type-Examination (module B) has been completed, the manufacturer may choose either the Production-Quality Assurance (module D), the Product-Quality Assurance (module E), or the Product Verification (module F), (in accordance with permitted modules in the MED Item List), based on its quality system and the level of implementation, and submit the application for the procedures chosen.
If the manufacturer has chosen the Unit Verification (module G), no further procedures other than this need to be chosen additionally. The inspections and auditing under the EC Type-Examination (module B), the Production-Quality Assurance (module D), the Product-Quality Assurance (module E), the Product Verification (module F) and the Unit Verification (module G) are briefly explained below:
EC Type-Examination (module B)
The Notified Body is to review the technical documentation for a specimen, conduct testing and inspections representative of the production envisaged, and ascertain and attest that the type complies with the provisions of the international instruments that apply to it. Once it has been ascertained that it complies with the provisions, the Notified Body is to issue an EC Type-Examination Certificate.
Production-Quality Assurance (module D)
The Notified Body is to conduct the auditing of the quality system for production, final-product inspection and testing for the products concerned of the manufacturer. Upon satisfactory completion of the auditing, the Notified Body is to issue an EC Notification of Compliance.
Then, the manufacturer should affix the Wheel Mark to each product and issue a Declaration of Conformity.
If the manufacturer has its quality management system certified by and registered with the Society with the applicable standard of ISO 9001 and with the audit scope covering the products concerned, it may be having an advantage when applying for the Production-Quality Assurance (module D), as it can demonstrate that it has an effective certified quality system.
Product-Quality Assurance (module E)
The Notified Body is to conduct the auditing of the quality system for final inspection and testing for the products concerned of the manufacturer. Upon satisfactory completion of the auditing, the Notified Body is to issue an EC Notification of Compliance. Then, the manufacturer should affix the Wheel Mark to each product and issue a Declaration of Conformity. If the manufacturer has its quality management system certified by and registered with the Society with the applicable standard of ISO 9001 and with the audit scope covering the products concerned, it may be having an advantage when applying for the Product-Quality Assurance (module E), as it can demonstrate that it has an effective certified quality system.
Product Verification (module F)
The Notified Body is to conduct the appropriate tests and examinations for every product and ascertain that they comply with the requirements of the international instruments. Upon satisfactory completion of the tests and examinations, the Notified Body is to issue an EC Certificate of Conformity.
Then, the manufacturer should affix the Wheel Mark to each product and issue a Declaration of Conformity.
Unit Verification (module G)
For products to which the EC Type-Examination (module B) is not appropriate to be applied, the conformity assessment procedures may be the Unit Verification (module G).
The Notified Body is to conduct the tests and examinations for the individual products and ascertain that they comply with the relevant requirements of the international instruments. Upon satisfactory completion of the tests and examinations, the Notified Body is to issue an EC Certificate of Conformity.
Then, the manufacturer should affix the Wheel Mark to the individual products and issue a Declaration of Conformity.
(2) Periodical Inspections and Surveillance Auditing
EC Type-Examination (module B)
The Notified Body is to issue an EC Type-Examination Certificate that is currently valid (maximum 5 years). At the request of the manufacturer, the certificate should be renewed for another 5 years after conducting tests and examinations as necessary.
Production-Quality Assurance (module D) and Product-Quality Assurance (module E)
Periodical surveillance is to be carried out to ascertain that the quality management system implemented by the manufacturer is maintained.
If the manufacturer has its quality management system certified by and registered with the Society with the applicable standard of ISO 9001 and with the audit scope covering the products concerned, it may be having an advantage when applying for the Production-Quality Assurance (module D) or the Product-Quality Assurance (module E) , as it can demonstrate that it has an effective certified quality system.
Regarding the periodical surveillance audits, the system generally follows the ISO 9001 certification in sequence.
Step 5. Affixing of Wheel Mark and Issue of Declaration of Conformity
After satisfactory completion of the inspections and auditing, the manufacturer should affix the Wheel Mark to the products, issue a Declaration of Conformity and ship the products with the Declaration.
Step 6. Important points of Directive 2014/90/EU
Please be aware of the following new requirements to the newly revised 2014/90/EU (enforced from 18 September 2016).
- A copy of the EU declaration of conformity shall be provided to the ship
- A copy of the EU declaration of conformity shall be provided to the notified body
- Appointment of Authorised Representative(applies to manufacturers located outside of the EU.)
Any Inquiries
For further information and/or for any inquiries including the procedures for inspections, auditing and certification, please contact the following locations:
Nippon Kaiji Kyokai
Piraeus Office
(Nippon Kaiji Kyokai (Greece) S. A. as Notified Body)
Tel: +30-210-483-2404Fax: +30-210-483-2405
e-mail: medgr@classnk.or.jp or pr@classnk.or.jp
Nippon Kaiji Kyokai
Materials and Equipment Department
(For EC Type-Examination (module B), Product Verification (module F) and Unit Verification (module G))
Tel: +81- 3-5226-2020Fax: +81- 3-5226-2057
e-mail: eqd@classnk.or.jp
Nippon Kaiji Kyokai
Certification Department
(For Production-Quality Assurance (module D) and Product-Quality Assurance (module E)
Tel: +81- 3-5226-2178Fax: +81- 3-5226-2179
e-mail: qad@classnk.or.jp